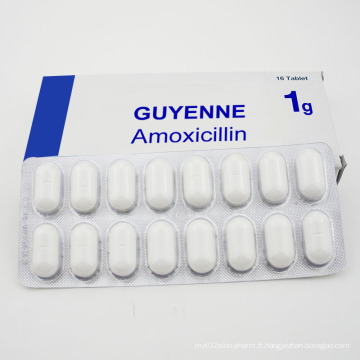
Amicicilline Ampiclox et Amoxici Tablet Llin
Informations de base
Modèle: Am-160629AMXL
Description du produit
N ° de Modèle: Am-160629AMXL Mode d'Emploi: Pour l'administration orale État: Solide Type: Produits Biologiques Marque: Guyenne Origine: France Application: Médecine interne Convient pour: Tous Forme: Tablette Technologie Pharmaceutique: Extraction de produit naturel Spécification: 250mg / 500mg Code HS : 2941109200 \ n  \ nDescription du produit \ nLes patients positifs à l'allergie à la pénicilline et à la pénicilline sont handicapés
\ nDescription du produit \ nLes patients positifs à l'allergie à la pénicilline et à la pénicilline sont handicapés
 \ nDescription du produit \ nLes patients positifs à l'allergie à la pénicilline et à la pénicilline sont handicapés
\ nDescription du produit \ nLes patients positifs à l'allergie à la pénicilline et à la pénicilline sont handicapés | Product: | tablet |
| Indications | Otitis media, sinusitis, pharyngitis, tonsillitis, urinary tract infections, skin and soft tissue infections, acute bronchitis, pneumonia, gonorrhea, typhoid, infectious diseases, sexually transmitted diseases, dermatology, renal medicine, respiratory medicine, ENT, purulent pleurisy, hepatobiliary system infections, sepsis, dysentery. |
| Dosage | Oral. 1, adults once 0.5g, every 6 to 8 hours a day dose does not exceed 4g. 2, children daily dose according to body weight 20 ~ 40mg / kg, every 8 hours; three months following the baby daily dose according to the weight 30mg / kg, every 12 hours. 3, patients with severe renal impairment need to adjust the dose, which endogenous creatinine clearance rate of 10 ~ 30ml / min in patients with 0.25 ~ 0.5g every 12 hours; patients with creatinine clearance less than 10ml / min 0.25 per 24 hours ~ 0.5g. |
| Adverse reactions | 1. Nausea, vomiting, diarrhea and pseudomembranous colitis and other gastrointestinal reactions. 2. Rash, drug fever and asthma and other allergic reactions. 3. Anemia, thrombocytopenia, eosinophilia cells. 4. Serum aminotransferase may be slightly increased. 5. Caused by the Candida or resistant superinfection. 6. Occasionally excitement, anxiety, insomnia, dizziness, and behavioral abnormalities, central nervous system symptoms. |
| Taboo | test-positive patients allergic to penicillin and penicillin skin disabled. |
| NOTES | 1. It must be done before using sodium penicillin skin test positive for the banned. 2. Infectious mononucleosis in patients with the FDA should be prone to rash, should be avoided. 3. Longer course of treatment patients should check liver and kidney function and blood. 4. It can lead to the use of Benedict or Fehling reagent false positive urine test. 5. It should be used with caution in the following cases: (1) have asthma, a history of hay fever and other allergic diseases. (2) may have to adjust the dose elderly and severe renal impairment |
Groupes de Produits : Antibiotiques
Premium Related Products
autres produits
Produits phares
Comprimés à 200 mg de chlorhydrate d'amiodarone de haute qualitéCrème de monobenzone de peau blancheMédecine générale Omeprazole 20mg Injection pour Gastrohelcosis et Stomach AcidMédicament général Ceftriaxone Sodium InjectionReady Stock Skin Whitening anti-âge Vitamine C InjectionTablette OEM 500mg ParacétamolHaute qualité 500mg Capsules rigides Amoxicillin (amoxycilline)Meilleur et bas prix Ceftriaxone Sodium Injection CeftriaxoneMeilleure qualité Omega 3 Deep Sea Softgel Capsule Huile de poissonArtemisinine antipaludique curative approuvée par la FDAMédecine traitant une lésion cérébrale Injection de Citicoline SodiumProtease Alpha Chymolase pour œdème inflammatoireMédecine générale Medroxyprogesterone Actate InjectionAmikacin Injection Médicaments généraux MédicamentsBlanchiment de la peau Gsh Soins personnels Glutathion InjectionBody Slimming Fitness Perdre du poids Perte de poids L-Carnitine Injection2.0g / 5ml